According to the latest updates from the National Medical Products Administration, the first domestically developed and innovatively antidepressant drug with independent intellectual property rights, Ruoxinlin®, was approved for market launch on November 3rd.

National Medical Products Administration Announcement [6]
According to the latest updates from the National Medical Products Administration, Ruoxinlin®, an antidepressant drug independently developed by Luye Pharma, specifically the Trudexta® extended-release tablets (venlafaxine hydrochloride), was approved for market launch on November 3rd. This marks the first domestically developed and innovatively antidepressant drug in China with independent intellectual property rights. [3]
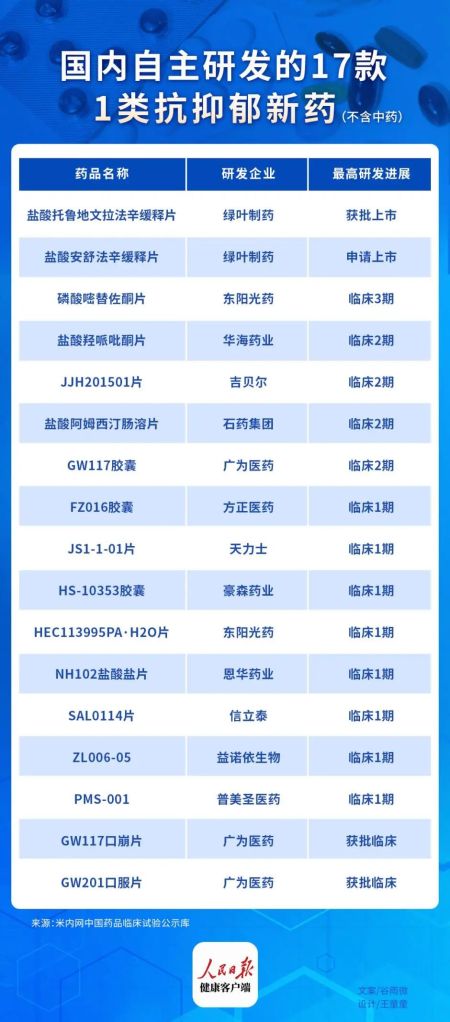
Traditionally, China relied on imported antidepressants, but now, Luye Pharma becomes the first to pass market approval among 17 domestically developed innovative antidepressants in various stages of research and development, according to incomplete data from the National Medical Products Administration. [1]
Reported by People’s Daily [1]
According to an official report from Luye Life Science Group, Ruoxinlin® is positioned as a comprehensive and stable treatment for depression. It aims to provide effective treatment without causing drowsiness and avoids negative impacts on sexual function, weight, and lipid metabolism. The report highlights its potential to address clinical needs unmet by many existing drugs, potentially improving the current treatment landscape for depression. [2]
The following is a brief overview of the research on Ruoxinlin from the official report of the Luye Group. “The mechanism of action study results for Ruoxinlin® have been published in ‘Frontiers in Pharmacology.’ Phase II clinical results have been published in the ‘International Journal of Neuropsychopharmacology’ and presented at the 19th National Congress of Psychiatry of the Chinese Medical Association. Phase III clinical results were released at the 2022 American Psychiatric Association Annual Meeting (APA).”
Preclinical mechanism studies have demonstrated that Ruoxinlin acts as a triple reuptake inhibitor (SNDRI) for serotonin (5-HT), norepinephrine (NE), and dopamine (DA). “5-HT, NE, and DA play crucial roles in the pathogenesis of depression within the nervous system.” [2]
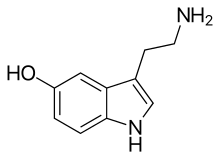
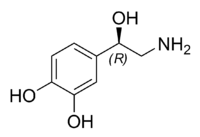
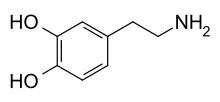
Diagram illustrating serotonin (5-HT), norepinephrine (NE), and dopamine (DA) [7]
In conclusion, the market launch of Ruoxinlin® represents a milestone in the development of antidepressant drugs in China and has the potential to reshape the market.
Speculative analysis anticipates that Luye Pharma, with the support of Ruoxinlin®, may secure substantial government backing, providing a significant advantage in the Chinese antidepressant drug market. This is considered a major positive development for Luye Pharma, and the drug’s prospects are promising.
References
[1] http://www.jksb.com.cn/index.php?m=content&c=index&a=show&catid=788&id=181950
[2] https://www.luye.com/info.php?id=447
[3] https://www.nmpa.gov.cn/yaowen/ypjgyw/20221103083801194.html
[4] https://mp.weixin.qq.com/s/_BvI4l9aDBU1Zf8OWqxB1Q
[5] https://mp.weixin.qq.com/s/De3RWqEj5S39Nx1plmo7MQ
[6] https://www.nmpa.gov.cn/yaowen/ypjgyw/20221103083801194.html
[7] https://en.wikipedia.org/wiki/Norepinephrine
In Transcription
@UCLA
封面|Unsplash @DeepMind
文案|杨中石 (Industry)
排版|Rita
校阅|Jasmine (Industry), Rita


Leave a comment